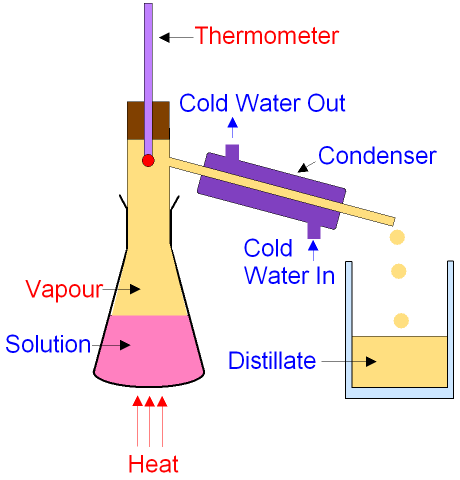
gcsescience.com 6 gcsescience.com
Elements, Compounds and Mixtures
What is Fractional Distillation?
Fractional
Distillation is a separation
technique that is used
for
liquids
that dissolve in
each other.
Liquids that dissolve
in each other are called miscible.
A liquid can be separated from a mixture
of liquids in a solution by fractional
distillation.

The solution is heated
until it boils.
The liquid with the lowest boiling point
boils first
and becomes a vapour (gas).
The vapour is cooled
in the condenser until the
temperature falls
below the boiling
point when it condenses
back into a liquid which is collected in a container.
The collected liquid is called the distillate.
It has been distilled.
The condenser has cold water running through
a jacket around the outside
to keep the
temperature below the boiling
point of the vapour.
After the liquid with the lowest boiling point
has
been collected,
the temperature of the remaining mixture
will
rise to a new
temperature
when
the liquid with the next
lowest boiling point
will
boil and be collected. The process can
be continued to
separate all the liquids in the mixture.
Fractional distillation
is used to separate
the components of crude oil
and to separate nitrogen
and oxygen from liquid air.
Immiscible liquids are separated using a separating funnel.
![]() Links
Solid
Liquid
Gas
Revision Questions
Links
Solid
Liquid
Gas
Revision Questions
![]()
gcsescience.com The Periodic Table Index Chemistry Quiz gcsescience.com
Home GCSE Chemistry GCSE Physics
Copyright © 2015 gcsescience.com. All Rights Reserved.