gcsescience.com 24 gcsescience.com
Straight Chain - Branched Chain - Boiling Point.
What is a Straight Chain?
A straight
chain is made from carbon atoms which are
joined onto no more than
two other carbon
atoms.
What is a Branched Chain?
A branched chain
is made
from carbon atoms
where at least
one carbon* atom
is joined onto
more than two other carbon
atoms.
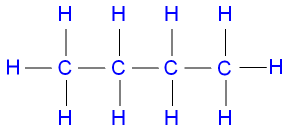
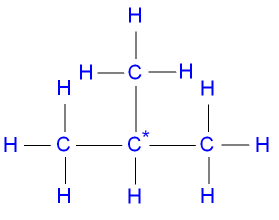
What are the Isomers of Butane?
Of the isomers of butane, n-butane has a
straight chain,

and 2-methylpropane has a
branched chain.
The two isomers have
different boiling points
(and other
physical properties).
n-butane
has a higher boiling point than 2-methylpropane.
What is the Effect of Chain Branching
on Boiling Point?
In general, straight
chain molecules have a higher
boiling
point than isomers with a
branched chain.
The reason is that straight
chain molecules can
line up beside each other
more effectively
than branched chain molecules and therefore
have a higher intermolecular force of attraction.
In addition to straight
chains and branched chains,
carbon atoms can form rings.
![]() Links Hydrocarbons Revision Questions
Links Hydrocarbons Revision Questions ![]()
gcsescience.com The Periodic Table Index Hydrocarbons Quiz gcsescience.com
Home GCSE Chemistry GCSE Physics
Copyright © 2015 gcsescience.com. All Rights Reserved.